Every year on November 14th, the bell for World Diabetes Day rings as scheduled, Continuous Glucose Monitoring (CGM) technology has become a key tool to solve the challenges of blood glucose management with its core advantages of “real-time, continuous, and accurate” monitoring. With the continuous technological progress of domestic CGM manufacturers, they have begun to actively expand into overseas markets. However, as new entrants, domestic CGM manufacturers are facing patent challenges from international giants.
On September 3rd, the UPC Hague Local Division officially opened the hearing on two Preliminary Injunction (PI) applications filed by Abbott against Sinocare, a Chinese company, and its European distributor partners. Prior to initiating the patent lawsuit against Sinocare, Abbott had launched a series of patent lawsuits against another Chinese manufacturer, SiBionics, in multiple European regions, and even applied for sales injunctions in some areas. A direct result of these lawsuits was that SiBionics delayed its European market expansion. Abbott’s actions remind us that true innovation must withstand the test of patents. For Chinese enterprises to truly gain a foothold in the global market, they must learn and apply the rules of the game regarding intellectual property.
As the first CDMO service enterprise focusing on the CGM field in China, Bioecare Technology has built technical barriers in core sensor materials and manufacturing based on its full-stack technical reserves. It has become a core partner for global CGM innovative enterprises. Through technology empowerment and patent avoidance, Bioecare provides one-stop services for domestic CGM enterprises from sensor design to mass production, promoting domestic CGM enterprises to achieve technological independence and global breakthroughs, and reshaping the global CGM industry competition pattern.
1. What is CGM CDMO Service?
CGM CDMO service is a professional CDMO service focusing on the field of continuous glucose monitoring devices. It specifically addresses the special challenges in the R&D and production of CGM products. The service covers the entire chain from core bioelectrode R&D, outer membrane material optimization, sensor structure design, to clinical trial sample production, cGMP-compliant mass production, and global registration support. It helps customers accelerate the technological maturity and market launch of CGM products. Among them, ISO 13485 certification, as a global universal quality management standard for the medical device industry, is a core indicator to measure the standardization and reliability of CDMO services, and is also the foundation for ensuring product safety and compliance.

2. Why Do Some Enterprises Choose CGM CDMO Services?
As the “gold standard” for diabetes management, the development of CGM involves four core challenges: sensor technology, algorithm optimization, large-scale production, and compliance. These challenges are intertwined, forming high industry barriers. By integrating full-chain capabilities in R&D, production, and compliance, CDMO provides enterprises with one-stop solutions to address the above challenges and accelerate product launch.
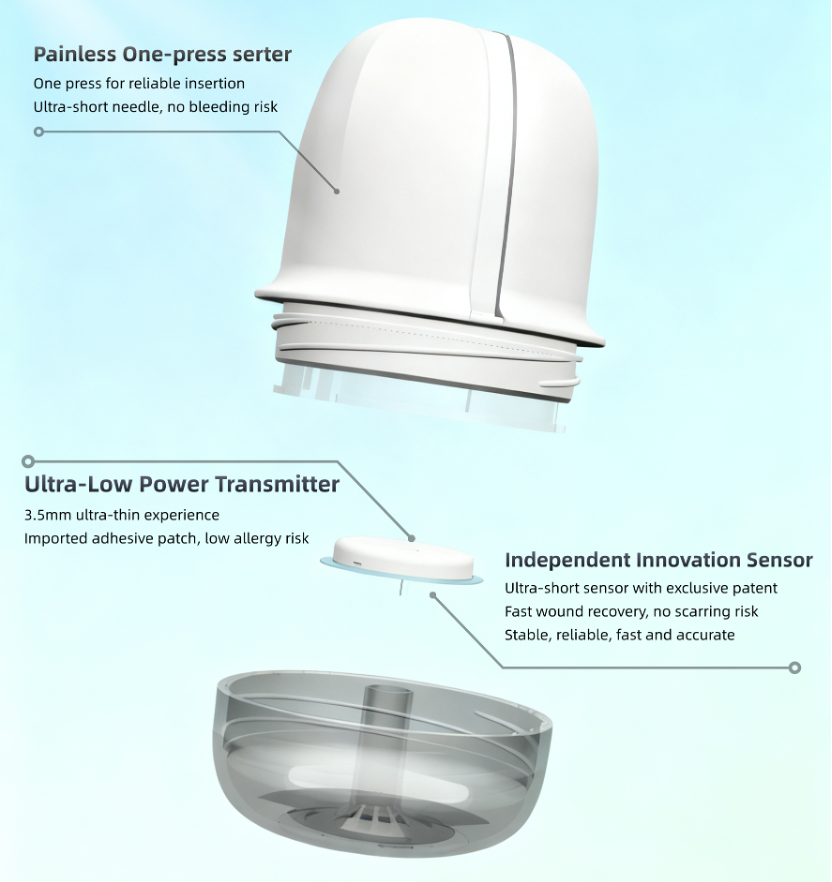
• CDMO enterprises possess core technologies in sensor design and manufacturing, helping enterprises solve issues such as miniaturization and biocompatibility.
• CDMO enterprises have algorithm development and optimization capabilities, assisting enterprises in resolving problems like signal processing and individual difference compensation.
• CDMO enterprises have large-scale production capabilities, helping enterprises overcome low yield and high cost issues.
• CDMO enterprises have compliance capabilities, aiding enterprises in handling regulatory approval matters.
• Through resource sharing and large-scale production, CDMO enterprises help enterprises reduce costs and risks.
3. Four Core Advantages of Bioecare Technology’s CGM CDMO Services
As a professional CDMO service provider in China’s CGM field, Bioecare Technology has formed unique competitive advantages distinct from general-purpose CDMO enterprises based on technical accumulation and industrial practice, providing customers with more targeted value empowerment.
3.1 Full-Stack Technical Barriers from Vertical Field Expertise
Construct a full-stack technical system covering “core materials - key components - intelligent manufacturing - regulatory compliance” to accurately address common industry challenges.

3.2 One-Stop Service Capabilities Throughout the Entire Lifecycle
Bioecare Technology has built an integrated full-lifecycle service system covering CGM products’ “R&D - production - compliance”, realizing full-process accompaniment from the initial R&D stage to the commercialization stage.
3.3 Large-Scale Adaptability of Technology and Production Capacity
From clinical samples to commercial mass production, CGM products face challenges in stability and cost control during process scaling. Through independently developed special production equipment and standardized processes, Bioecare Technology achieves precise transfer of process parameters.
3.4 Customized Service Model with Deep Binding
Bioecare Technology adopts a customized cooperation model with in-depth customer collaboration. Leveraging its experience in overseas business layout, it provides global market access support to help technologies go global.
4. Which Enterprises Are Suitable for CGM CDMO Services?
The core value of CGM CDMO services lies in helping enterprises avoid R&D risks, reduce production capacity investment, and accelerate the market launch process. It is particularly suitable for the following entities:
4.1 Mature Enterprises with Cross-Border Layout
Although mature enterprises with cross-border layout have advantages in capital and market, they lack technical accumulation and industry experience in the CGM field. Bioecare Technology can provide full-process support from technical research to product launch, helping enterprises quickly achieve cross-border layout.
Bioecare Service Case: Empowering Brands to Seize the Pet CGM Opportunity
A company focusing on pet health products has pet health channel resources but completely lacks CGM technology R&D and production experience. Independent development faces multiple technical challenges such as animal physiological adaptability and detection range matching. Bioecare Technology tailored a full-process solution of “pet-specific development + rapid verification + mass production launch” for it. Considering the physiological characteristics of dogs and cats with wider blood glucose fluctuations, it optimized the electrode sensing system to reduce implantation discomfort and shedding risks. Relying on this pet-specific CGM product, the company successfully seized the blue ocean market of precise pet blood glucose monitoring and became a first-mover brand in this niche field.
4.2 Established Medical Enterprises with Upgrade Needs
Bioecare Technology can help enterprises break through technical bottlenecks with its technological innovation and process optimization capabilities. Meanwhile, it optimizes the cost structure with large-scale production experience to enhance product competitiveness.
Bioecare Service Case: Assisting Established Enterprises in Technical Iteration and Market Competitiveness Upgrade
An established medical enterprise with more than 20 years of experience in the blood glucose monitoring field has a stable market share with its traditional blood glucose meter products. However, in the CGM track, it is limited by existing technical paths and production equipment, and core challenges such as electrode stability and outer membrane durability have not been resolved. Bioecare Technology set up a special technical team to assist its transformation from a traditional blood glucose meter enterprise to the CGM market.
4.3 Technology-Driven Startups
By choosing Bioecare Technology’s CDMO services, startups can directly reuse mature technical platforms and production facilities, focus their core energy on technological iteration and market expansion, and significantly reduce initial investment and trial-and-error costs.
Bioecare Service Case: Empowering Startups to Achieve Rapid Breakthroughs from Technical Concepts to Clinical Samples
A startup focusing on wearable medical devices has CGM algorithm and software technology but lacks sensor hardware R&D and production capabilities. Since CGM sensors involve multiple technical links such as bioelectrode preparation and outer membrane material modification, establishing an in-house team and production line would require an investment of over 10 million yuan and a cycle of more than 2 years, which exceeds the affordability of startups. Bioecare Technology customized a phased service plan of “core component development + process verification + sample production” for it. Currently, the enterprise has launched clinical pre-trials with this sample and successfully obtained a new round of financing.
5. Future Outlook: Technology Integration Empowers a New Ecosystem of Precision Medicine
In the future, Bioecare Technology will continue to focus on the integrated innovation of advanced packaging, intelligent sensing technology, and advanced semiconductor technology. Combined with artificial intelligence technology, it will promote the upgrading of CGM products towards more accurate, intelligent, and convenient directions. In terms of CDMO service capability building, it will further expand technical service capabilities in related fields such as in vitro diagnostics, neuromodulation, and wearable health, constructing a broader bioelectronic technology service ecosystem. From precision medicine to general health, and from monitoring to treatment, Bioecare Technology aims to become a core hub connecting technological innovation and clinical application.